Rv0534c - 1,4-dihydroxy-2-naphthoate octaprenyltransferase menA
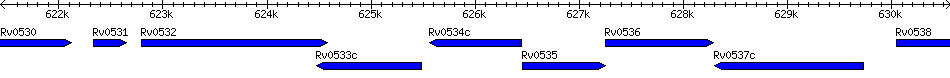
|
|
Protein Domains  |
| Gene Information | |
|---|---|
| Locus | Rv0534c |
| Symbol | menA |
| Gene Name | 1,4-dihydroxy-2-naphthoate octaprenyltransferase menA |
| Location | 625562 - 626440 (-) |
| Species | Mycobacterium tuberculosis H37Rv complete genome. |
| Length | Gene:879 bp Protein:293 aa |
| External Links | Tuberculist Target Gene Information String Protein-Protein Interactions STITCH Chemical-Protein Interactions Search Google Scholar |
Orthologs

|
| Orthogroup Number | 18777 |
| Related Genes | Acel_0150 BL1655 CE0469 cg0531 DIP0418 jk1877 MAP4029c MAV_4611 Mkms_0716 ML2406 Mmcs_0702 MSMEG_0988 MT0558 MUL_0633 Mvan_0880 nfa51470 PPA1945 | Transcriptional Regulation |
| Operons | View gene in operon browser |
| Regulatory Network | |
| Expression Correlation |
Genes with Correlated Expression Scatterplot of Gene Expression |