Rv2592c - holliday junction DNA helicase ruvB

|
|
Protein Domains 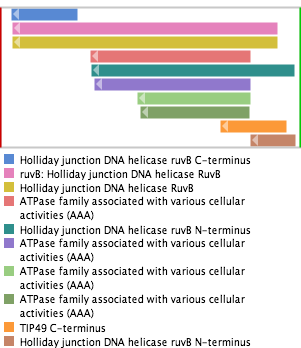 |
| Gene Information | |
|---|---|
| Locus | Rv2592c |
| Symbol | ruvB |
| Gene Name | holliday junction DNA helicase ruvB |
| Location | 2923199 - 2924233 (-) |
| Species | Mycobacterium tuberculosis H37Rv complete genome. |
| Length | Gene:1035 bp Protein:345 aa |
| External Links | Tuberculist Target Gene Information String Protein-Protein Interactions STITCH Chemical-Protein Interactions Search Google Scholar |
Orthologs

|
| Orthogroup Number | 712 |
| Related Genes | Acel_1343 BL0729 CE1773 cg1869 DIP1375 jk1054 MAP1038 MAV_3473 Mkms_2314 ML0483 Mmcs_2267 MSMEG_2945 MT2669 MUL_1716 Mvan_2575 nfa36950 PPA1160 SAV6835 | Transcriptional Regulation |
| Operons | View gene in operon browser |
| Regulatory Network | |
| Expression Correlation |
Genes with Correlated Expression Scatterplot of Gene Expression |
| |||
| Proteins | |||
|---|---|---|---|
| Genomic Sequence | |||